Research
February 1, 2021 2023-08-02 15:55Research
Overview
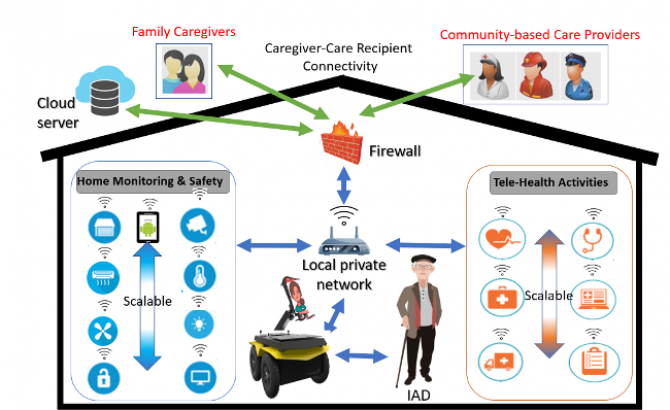
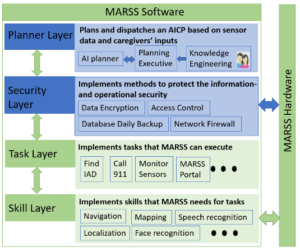
The MARSS framework has four evidence-supported modalities of AD care: activity engagement and assistance, telehealth, home safety, and caregiver-care recipient connectivity. We also have a training program to enable a non-technology expert (caregiver, family member or health professional) to scale and program the modalities to fit with the disease severity and context of the IAD.
MARSS is being developed and tested in accordance with the NIH Stage Model of Intervention Development. Stage I and II involve scaling up the existing lab-based model of MARSS for a pilot test in the community (with 8 dyads of IAD and caregivers) and verify its implementation fidelity, robustness as well as behavior change techniques to optimize target engagement of IADs and caregivers (2022-2024). Stage III involves an 18-month randomized controlled trial to validate the real-world efficacy of MARSS (2025-2027). We will recruit 60 dyads in two staggered cohorts of IAD and caregivers and randomly assign them to the intervention (n=30) or a control group (n=30). We will gather repeated measures data on the IAD’s functional independence, safety, and physical and cognitive health, and the caregiver’s perceived care burden, autonomy and wellbeing over nine data points, 2 months apart. To account mechanism-focused change, we will objectively collect data on the technology’s utilization to tease out the influence of the MARSS’s modalities on the intervention outcomes.